X-rays Computed Tomography (CT)
X-rays Computed Tomography (CT) makes use of X-ray images (projections) taken from different angles and image reconstruction techniques to produce anatomic cross-sectional (tomographic) 3D images. CT contrast agents (usually based on Iodine, Barium, Gold) can be used to enhance the contrast.
In microCT, the instrumentation is implemented with accessories or with a technical set-up which provides optimum resolution and sensitivity for small animal studies.
The spatial resolution of microCT is higher with respect to clinical CT and typically the voxel size is lower than 100 mm for in vivo imaging and even smaller (less than 10 mm) for ex-vivo sample imaging. In order to achieve high spatial resolution a microfocus X-ray source is installed on microCT scanners.
Another difference with respect to clinical CT scanners is the use of flat panel detectors (FPD) with small pixel sizes (less than 100 mm) instead of curved detectors arrays. The use of a FPD results in a cone beam acquisition geometry. In this case, dedicated image reconstruction algorithms, such as the Feldkamp filtered back projection (FBP) algorithm, are needed.
Preclinical microCT systems can be stand alone or integrated with other modalities like optical or nuclear medicine imaging in order to perform multimodal imaging. microCT devices can be also found in small animal radiotherapy systems for animal positioning and treatment planning.
At Euro-BioImaging, preclinical and ex-vivo CT is provided by the following Nodes:
- Austrian BioImaging / CMI (AT)
- Center for Advanced Preclinical Imaging (CAPI) (CZ)
- Digital Imaging Multimodal Platform Neuromed - DIMP NEUROMED
- Danish BioImaging (DK)
- Facility of Multimodal Imaging - AMMI Maastricht (NL)
- Finnish Biomedical Imaging Node (FI)
- Flanders BioImaging Node (BE)
- Hungary: Medical and Preclinical Imaging Hungary (HU)
- Israel BioImaging (IL)
- Molecular Imaging Italian Node (IT)
- Preclinical Imaging Centre (PRIME) - Molecular Imaging Dutch Node
- Swedish NMI (SE)
Use cases
Click here for Use Cases from our Nodes.
Dual energy CT (DECT) was firstly introduced in 1976 with the main goal of obtaining the photoelectric and Compton components of the absorption coefficients using a polychromatic x-ray beam with different energies. These two components are respectively proportional to atomic number (Z) and density of the material (r) (R.E. Alvarez and E. Macovski, “Energy-selective reconstructions in X-ray computerized tomography”, https://doi.org/10.1088/0031-9155/21/5/002).
By using DECT acquisitions it is thus possible to obtain rZ maps to gain material-specific information at each voxel as shown in the figure below.
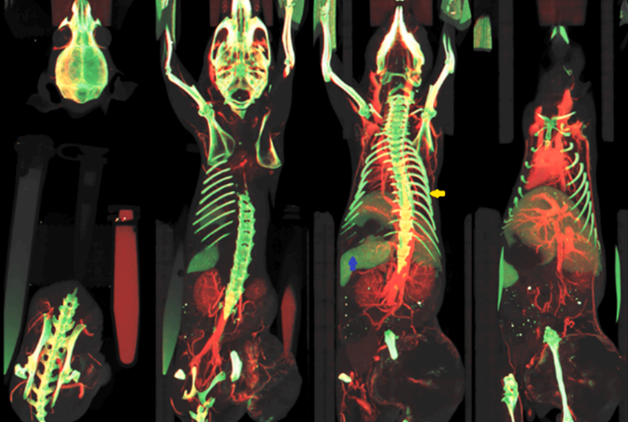
A significant boost of DECT came in 2006 with the introduction of dual source CT. In this case two x-ray tubes operating at different tension are orthogonally mounted in the gantry and both high and low tension images are acquired (axial or spiral mode) at the same time, reducing artifacts induced by movement (T.G. Flohr et al. “First performance evaluation of a dual-source CT (DSCT) system”, https://doi.org/10.1007/s00330-005-2919-2).
Current CT scanners are mostly based on scintillator energy integrating detectors and it is thus not possible to gain information about the energy of the detected x-ray photons. Photons counting detectors (PCD) were firstly introduced for nuclear medicine imaging modalities and their application for x-ray CT was limited by the low detector rate PCD can handle. However, considering also the developments of DECT, there has been a significant research interest in improving their material composition and reading electronics.
A PCD is made of a semiconductor (e.g. Cadmium Telluride) where the interaction with the incoming x-ray generates positive and negative charges proportional to the energy of the incoming photons. Different signal thresholds corresponding to different energy values can be set in order to divide the detected transmitted x-ray spectrum into different energy bins.
The use of PCD is particularly important for DECT as shown in the figure below, where DECT imaging of a mouse using PCD is presented. In this example, material decomposition into three basis materials was performed.
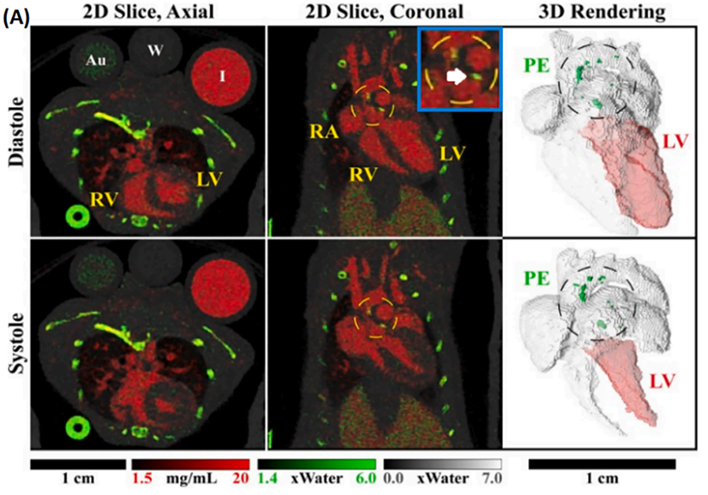
In subtraction X-ray imaging, tissue structures or organs are visualized using a contrast medium and measuring the changes in the attenuation between the contrasted structure and the surrounding tissue. In K-edge subtraction (KES) imaging, two X-ray images are taken at different mean energies, slightly below and a bit above the K-edge of the contrast agent photoelectric absorption. Their subtraction generates an image only displaying the contrasted structure.
So far, this method mostly relies on monochromatic X-rays produced at large synchrotron facilities.
KES allows to differentiate similarly absorbing substances in contrast enhanced CT, such as for example commonly used iodine contrast agents and calcium which is typically seen in calcifications, kidney stones and bones.
One of the main limitations of CT is the poor contrast of low Z materials, e.g. soft tissues, because of similar X-ray absorption. In order to solve this problem the diffraction and refraction of the X-rays can be exploited to obtain more information about the structure of the object. This technique is called Phase Contrast Imaging. See Phase Contrast CT Imaging for more information here.