SPAIN
Barcelona Mesoscopic Imaging Node (BMIN)
BMIN is a multi-sited, single-technology Flagship EuroBioimaging Node, with two access sites located in the area of Barcelona, at IRB Barcelona (Institute for Research in Biomedicine) and ICFO (Institute of Photonics Science). It offers open access to a wide range of mesoscopic imaging modalities, mostly based on Lightsheet Fluorescence Microscopy (LSFM) customized and unique instrumentation, for multiscale optical imaging spanning from 50µm to over 5 cm samples size. BMIN applications include the observation of living organisms, at fast imaging pace, great depth and “all around” (e.g. multiview), or imaging whole organs and organisms in toto with cellular resolution.
Specialties and expertise of the Node
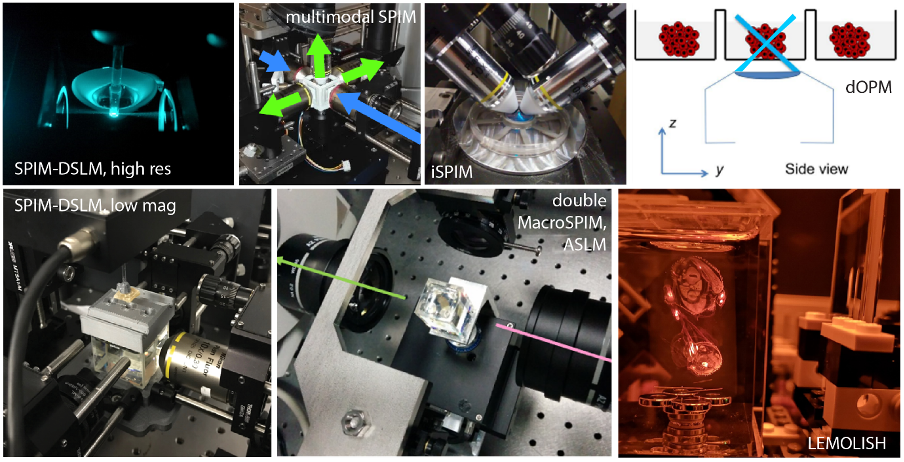
The imaging modalities are centrally devoted to multimodal lightsheet imaging (e.g. SPIM, DSLM, ASLM, iSPIM, OPM, “scattered” sLS, and more), which can be also combined with other complementary technologies for correlative workflows. BMIN aims to provide life scientists in the public and private sectors with full imaging workflows, including project’s definition and supervision, sample preparation, image data acquisition, bioimage analysis and visualization, and temporary data storage.
LSFM enables either fast, gentle and/or large volume imaging with samples ranging from mid-sized organoids/spheroids up to embryos or entire organisms. Both living samples and fixed tissues can be imaged under LSFM with great benefits compared to conventional point-scanning 3D imaging techniques (e.g. confocal). At BMIN, seven custom instruments are designed to enhance specific imaging features or performance tailored to either the samples’ size or its longevity. Our strength stands in our ability to pick, or develop/adapt, the optical configuration that best suits the biological question, and in cases to tackle multiscale imaging with the combined use of several instruments.
Here below, we review the features and advantages of the Lightsheet variants implemented at the Node’s instruments.
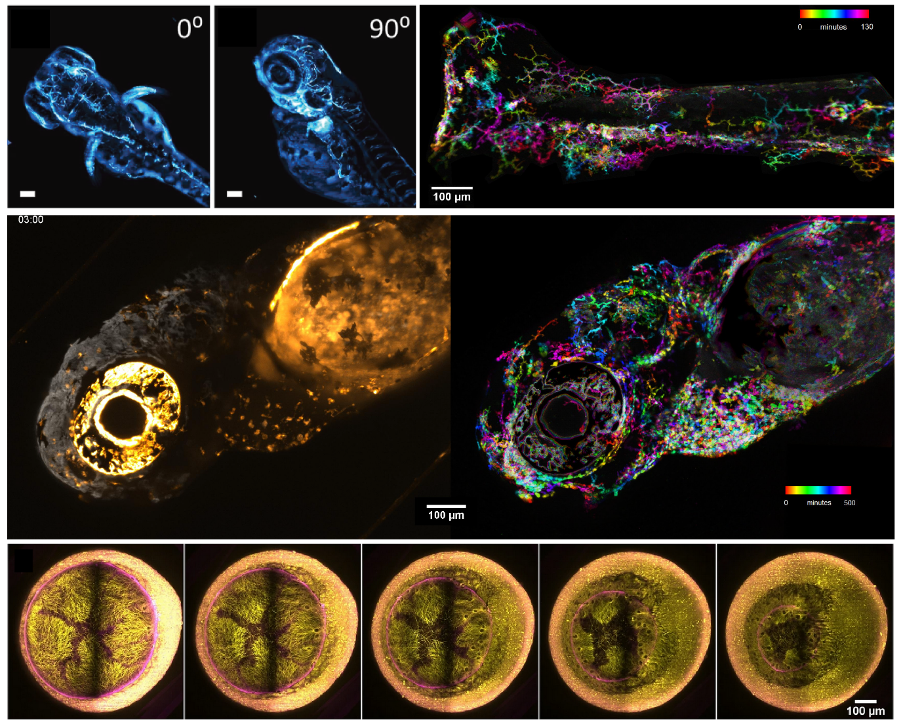
Lightsheet (LS) variants and features available for multimodal live imaging. “SPIM”: Selective Plane Illumination Microscopy. With a static LS illumination, SPIM enables deep, fast and gentle imaging. Commonly set with a horizontal detection arm and vertical ligthsheet illumination, it offers the simplest and most flexible readout for samples hanging from the top or disposed in a cuvette or chamber.
Multiview SPIM: Most instruments enable sample rotation, hence enabling one to access the sample from the best angle, or to generate a more homogeneous and more isotropic 3D volume in large samples after multiview reconstruction.
Double-sided illumination:For large samples, two lightsheets are used, e.g. from “right” and from “left”, and can be combined to improve the image quality over large fields of view
Double-sided detection: Imaging large samples from both sides simultaneously enables sharper images with large samples. With live samples it results in faster imaging (no rotation), with cleared samples it extends the size of the sample that can be imaged.
DSLM: Digitally Scanned Lightsheet Microscopy. The lightsheet is produced by a rapidly scanned beam (in one direction only) to form a LS that yields improved image contrast, quality and resolution. Especially suited to work in the two-photon regime.
ASLM: Axially Swept Lightsheet Microscopy. While in SPIM, adjusting the LS focus to the field of view brings a known resolution trade-off, ASLM enables one to sweep the LS waist laterally to achieve the best possible axial resolution across the entire (or larger) field of view.
iSPIM: An inverted SPIM. The illumination and detection objective lenses reach the sample from the top (e.g. by dipping). This inverted geometry allows for easy sample mounting in dish-like or chamber-slide sample carriers.
Refocused imaging: This is either a SPIM or DSLM add-on based on active optics that adjusts the image plane across large volume without moving any part. By moving both the lightsheet and the focus optically, rather than the sample mechanically, one achieves fast volumetric imaging of living samples, reaching up to several tens of volumes per second.
OPM, Oblique Plane Microscopy: the lightsheet is formed through the same detection lens and the image is reconstituted through an optical assembly downstream of the primary objective lens. Mounted on an inverted microscope, OPM enables to combine the lightsheet benefits with the use of conventional sample carriers (e.g. multiwell plates), hence true high-throughput and long-term live imaging.
MacroSPIM with optical clearing: For large samples in the order of 5-30mm, imaging with cellular resolution in entire organs at variable magnifications (i.e. continuous zoom) ranging from 0.3x to 24x. Illumination and detection from both sides ease the capture of high contrast images in very large volumes. 3D Tiling enables good resolution over large volumes.
Diverging LS: A diverging lightsheet fills a very extended field of view and offers to image exceptionally large sample (5-7cm or beyond) in a single scan. Typically suited to late development (e.g. late chicken embryo) or full organisms.
sLS: Scattered ligthsheet.A label free modality that captures the elastic scattering of the laser through the sample to reveal specific scatterers, e.g. nanoparticles, different tissues composition or fibers in tissues
Sample mounting expertise: Depending on the instrument and application, we can employ several strategies to mount and image the sample:
Embedding in agarose: top-mounted for live imaging or cleared inside the agarose block.
In conventional sample carriers: slide, chamber-slide, petri-dish, multiwell-plates.
Resting in a custom cuvette
Fluidic approach: imaging samples flowing through a tube enables high-throughput imaging, turning a SPIM into a 3D image-based flow cytometer.
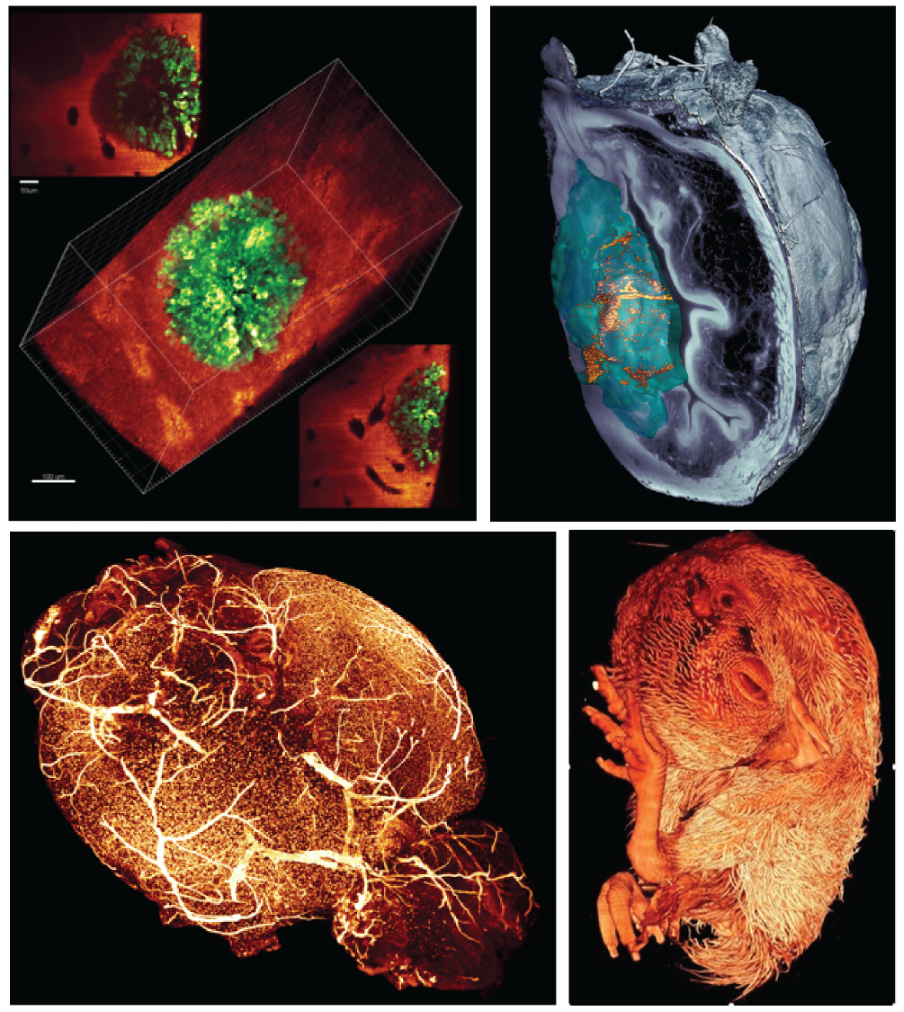
Label free capabilities: Through cleared tissues, autofluorescence and scattered lightsheet imaging can provide additional and complementary channels for tissue imaging. Using non-fluorescent elastic scattering of the lightsheet illumination, macroscopic structures or exogenous targets (e.g. nanoparticles) can be revealed at very low laser power levels.
Large data image analysis: The Node develops tools to enable large image data (>TBs) processing of lightsheet data, including tiled images stitching, multiview fusions, live data analysis, cell tracking.
Complementary imaging modalities: Whether to check the sample at higher resolution or to perform correlative imaging workflows, the Node offers alternative modalities to be used in combination with lightsheet imaging, including confocal microscopy, non linear microscopy, super resolution microscopy, Raman microscopy histological sectioning and imaging, etc.Offered Technologies
Lightsheet Mesoscopic Imaging (SPIM /sDSLM)
Tissue Clearing*
Expansion Microscopy*
High-Speed Imaging*
Feedback Microscopy*
Laser scanning confocal microscopy (LSCM/CSLM)
High Throughput Microscopy / High-Content Screening (HTM/HCS)
Fluorescence Lifetime Imaging (FLIM)
Two-Photon microscopy (2P)
Second/Third Harmonic Generation
Raman Spectroscopy
Image analysis -bio (IA-bio)*
Additional Services offered by the Node
Project planning and methodological setup (e.g. design of study protocol and standard operation procedures)
Instruments
Training in sample preparation and mounting
Customization of sample mounting
Technical assistance to run instrument
Probe preparation
Animal facilities (Drosophila)
Wet lab space
Bioimage Data processing, analysis and visualization facilities
Bioimage Data storage
Meeting and training seminar rooms
Administrative support for housing
Training in infrastructure use
Training workstations
Biological material storage and processing
Training in techniques for optical clearing of biological samples
Workshop services for mechanical prototyping (metal, 3D printed), electronics
Histology services (separate service) for correlative imaging after lightsheet imaging
Instrument highlights
DSLM with Two-photon (2P) excitation,
Multiview SPIM imaging,
DSLM with dual sided illumination, ASLM for high resolution across large field of view, and all optical refocusing for fast volumetric imaging,
Macro DSLM: DSLM at low magnification for 0.5-1cm cleared tissues,
MacroSPIM: SPIM with ASLM at low magnification for 0.5-3cm cleared tissues, dual side illumination dual side collection,
LEMOLISH: SPIM at very low magnification for 1-5cm cleared tissues, single-side illumination, dual side collection,
SPIM with fluidics sample mounting for High-throughput Imaging
SPIM for in vivo imaging and/or with fluidic sample mounting dual side illumination, dual side collection,
SPIM for fast volumetric imaging, incorporating electrically tunable lenses or adaptive optics,
Scattered Lightsheet label-free imaging available for cleared and living samples,
Oblique Plane Microscopy (dOPM): lightsheet on an inverted microscope for High-Throughput Live Imaging, dual view.
Contact details
Julien Colombelli: Head of Advanced Digital Microscopy Core Facility
Institute for Research in Biomedicine - IRB Barcelona
julien.colombelli@irbbarcelona.org
Pablo Loza-Alvarez: Head of Super-resolution Light Microscopy and Nanoscopy Laboratory (SLN)
Institute of Photonic Sciences – ICFO