How imaging technologies contribute to understanding viruses
Published: 2021-07-21
Euro-BioImaging offers an ever-expanding portfolio of cutting-edge imaging technologies and expertise that covers a range of applications in the life sciences – including in virology.
A number of Euro-BioImaging facilities are connected to strong local research expertise in virology, where they constantly work to support their local researchers in understanding all aspects of the biology of viruses and viral infections, from how viruses can enter and proliferate in their host cells to how the immune system responds to such infections. This research is often connected to the development of novel imaging technologies or applications, which are originally developed for the targeted research on a certain virus but can be efficiently adapted to new arising virus threats. The 137 imaging facilities across Europe that belong to the Euro-BioImaging family are happy to support your virus-related research project, and can advise on the most appropriate imaging technology to carry out your work. In addition, Euro-BioImaging currently offers Fast Track access to our infrastructure for COVID-19 related projects.
How can Euro-BioImaging facilities support your research on viruses?
Understanding the virus life cycle
Here's an example from the French BioImaging Node of Euro-BioImaging where novel methods are being developed to study different aspects of the virus life cycle. For instance, a study supported by the Bretagne-Loire site of the French BioImaging Node, used fluorescence lifetime imaging (FLIM) and fluorescence correlation spectroscopy (FCS) to elucidate the dynamics of virus particle assembly in living cells infected with the Hepatitis B virus [1]. Another study, a collaborative project between the Paris Centre and Bretagne-Loire sites of the French BioImaging Node and the Diamond Lightsource UK, developed an integrated, user-friendly platform for 3D correlative imaging of cells infected with the reovirus. This platform combined cryo-SIM (cryo-structured illumination microscopy), which allows super-resolution fluorescence imaging in cryo-samples, with soft X-ray tomography. Combining these methods allowed researchers to investigate the release pathway of the virus from its host cell [2].
Certain viruses, such as Influenza A, consist of multiple RNA pieces that have to correctly assemble into the virus particle in infected cells. The components necessary for this assembly were recently investigated by researchers at the Portuguese Platform of BioImaging (PPBI) Node using live cell imaging in combination with Fluorescence Recovery after Photobleaching (FRAP) as well as correlative light and electron microscopy. Their work showed that the viral inclusions in infected cells are membrane-less organelles and that they do not promote an escape from the interferon antiviral response [3].
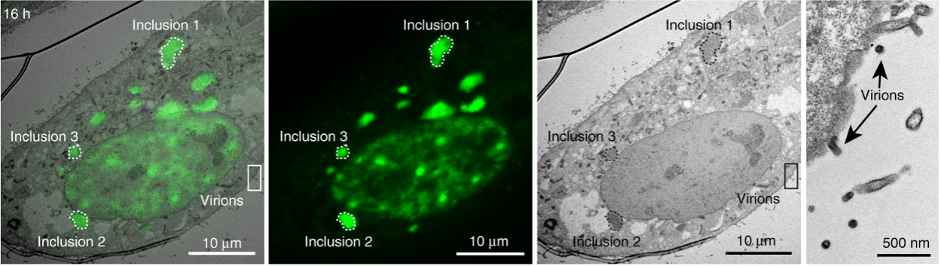
A virus-infected cell imaged with both confocal (2nd left) and electron microscopy (2nd right) and the overlay of the two images (left) as well as a zoomed in region (right). The cell shows multiple viral inclusions and virions budding from the surface. Image CC-BY-NC-ND 4.0 from [3]
Understanding viral infection
During the COVID-19 emergency, many of the Euro-BioImaging facilities demonstrated their ability to pivot their capacities and put them in the service of emergency research on pandemic threats, even under highly challenging conditions, such as during lockdowns with limited access to the facilities for staff and researchers. For example the Electron Microscopy Core Facility at the EMBL Node immediately at the start of the COVID-19 pandemic began a project in collaboration with researchers from the University of Heidelberg to understand how host cell physiology is affected by SARS-CoV2 infection [4]. This study combined light and electron microscopy to provide a comprehensive overview of the morphological changes in the cell’s organelles following a SARS-CoV-2 infection. The EMBL Node’s contribution was to use different EM techniques to visualize the steps in the virus replication process, including using FIB-SEM to study full cells in 3D at high resolution.
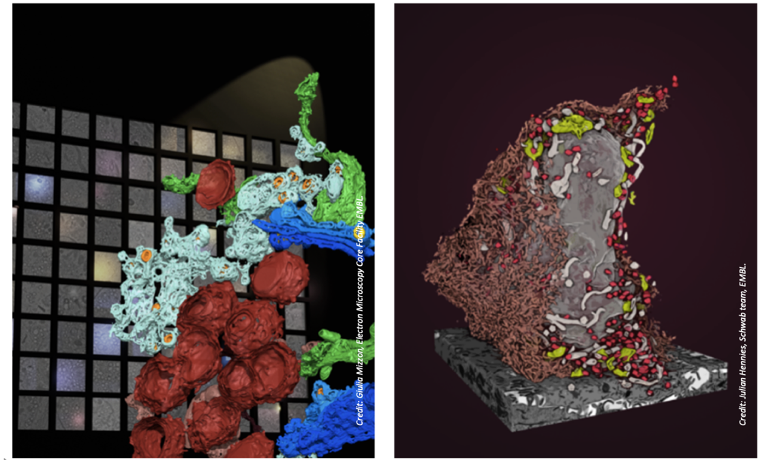
Left: 3D rendering of organelles visualised by TEM tomography. Multiple tomograms are accessible via an online browser. Credit: Giulia Mizzon, Electron Microscopy Core Facility EMBL. Right: FIB-SEM imaging reveals in 3D the ultrastructure of an entire infected cell. Credit: Julian Hennies, Schwab team, EMBL
Diagnostics and prognosis
Imaging not only plays a key role in understanding the biology underlying viral infections, it is also crucial in diagnostics and prognosis. The development of rapid and highly accurate diagnostics is one of the key challenges in a pandemic response and high-throughput imaging in combination with automated image analysis can be a key part of the solution. This method combination can bring higher specificity and broader coverage than classic ELISA-based diagnostic tests. Researchers from the Advanced Light Microscopy Facility at Euro-BioImaging's EMBL Node contributed to a study demonstrating the potential of this approach for COVID-19 detection by developing an image analysis pipeline capable of fast and specific detection of all three major classes of antibodies against COVID-19 in blood samples [5].
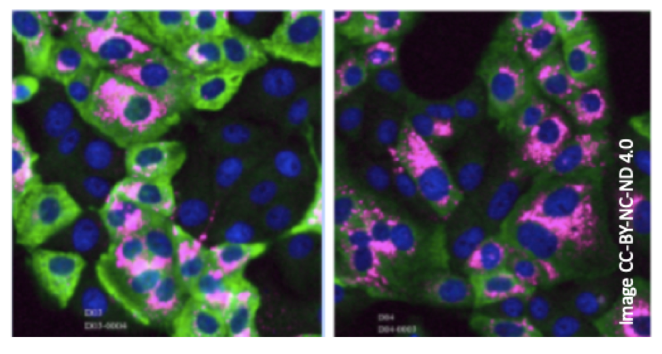
Above: Virus-infected cells employed in assays to test for the presence of SARS-CoV2-specific antibodies in human blood samples. Example images with immunolabeled SARS-CoV2-infected human cells showing the blood serum response of four different persons to the virus infection (Pink: virus RNA; Green: IgG antibody; Blue: Cell Nuclei). Image CC-BY-NC-ND 4.0
How human tissues are affected by infection
Understanding the effect SARS-CoV-2 infection has on the infected tissues is an important part of long-term patient care strategy development. Imaging plays a key role in this clinical research, as demonstrated by research supported by the Phase Contrast Imaging Flagship Node of Euro-BioImaging, based at the Elettra Synchrotron in Trieste, Italy, where 3D volume renderings of human lung specimens, including from COVID-19 patients, were obtained to allow for a comparison with clinical CTs and histo-pathological findings.
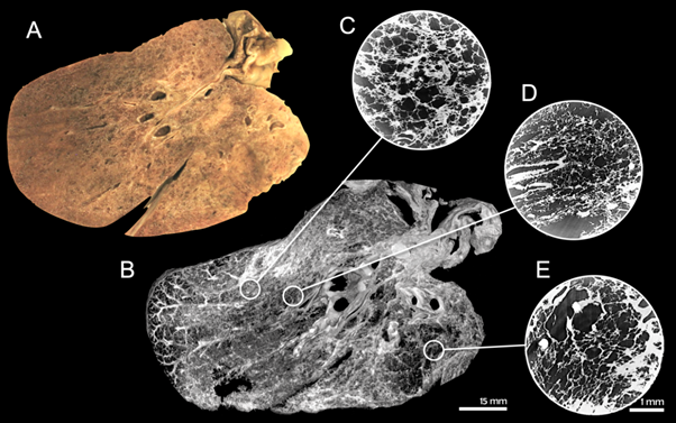
Photo of a lamella prepared for the study (A), a reconstructed slice of the entire tissue specimen at a resolution of 100 µm (B) and the outcomes of three local area scans performed in three selected areas (C-E) with a resolution of 5 µm
Predicting disease progression
CT scans of the lungs of SARS-CoV2-infected patients can help doctors to predict which patients will develop the most severe forms of the disease and therefore plan treatments accordingly. Researchers at the San Raffaele Hospital in Milan, part of the Euro-BioImaging Molecular Imaging Italian Node, developed artificial intelligence tools for the analysis of these CT scans and other clinical data to predict disease progression and help doctors prevent sudden deterioration of patient health through early treatment. The developed tool AI-SCoRE supports the stratification of health risk which has critical applications also for future epidemics and pandemics.
About Euro-BioImaging
Euro-BioImaging is the European landmark research infrastructure for biological and biomedical imaging as recognised by the European Strategy Forum on Research Infrastructures (ESFRI). Through Euro-BioImaging, life scientists can access imaging instruments, expertise, training opportunities and data management services that they might not find at their home institutions or among their collaboration partners. All scientists, regardless of their affiliation, area of expertise or field of activity can benefit from these pan-European open access services, which are provided with high quality standards by leading imaging facilities.
References
[1] Rat et al., Hepatitis B Virus Core Protein Domains Essential for Viral Capsid Assembly in a Cellular Context. J Mol Biol. 2020 Jun 12;432(13):3802-3819. doi: 10.1016/j.jmb.2020.04.026. Epub 2020 May 1. PMID: 32371046.
[2] Kounatidis et al., 3D Correlative Cryo-Structured Illumination Fluorescence and Soft X-ray Microscopy Elucidates Reovirus Intracellular Release Pathway, Cell, 2020 doi:10.1016/j.cell.2020.05.051
[3] Alenquer et al., Influenza A virus ribonucleoproteins form liquid organelles at endoplasmic reticulum exit sites, Nature Communications, 2019 doi.org/10.1038/s41467-019-09549-4
[4] Cortese et al., Integrative Imaging Reveals SARS-CoV-2-Induced Reshaping of Subcellular Morphologies, Cell Host & Microbe, 2020, doi:10.1016/j.chom.2020.11.003
[5] Pape et al. Microscopy-based assay for semi-quantitative detection of SARS-CoV-2 specific antibodies in human sera, BioEssays, 2020. doi: 10.1002/bies.202000257